Welcome to DU!
The truly grassroots left-of-center political community where regular people, not algorithms, drive the discussions and set the standards.
Join the community:
Create a free account
Support DU (and get rid of ads!):
Become a Star Member
Latest Breaking News
Editorials & Other Articles
General Discussion
The DU Lounge
All Forums
Issue Forums
Culture Forums
Alliance Forums
Region Forums
Support Forums
Help & Search
Women's Rights & Issues
Related: About this forumThis FDA Decision Could Transform Menopause Care
This FDA Decision Could Transform Menopause Care
PUBLISHED 11/12/2025 by Sharon Malone and Jennifer Weiss-Wolf
The end of the FDA’s “black box” era for estrogen could reshape conversations between women and their doctors.
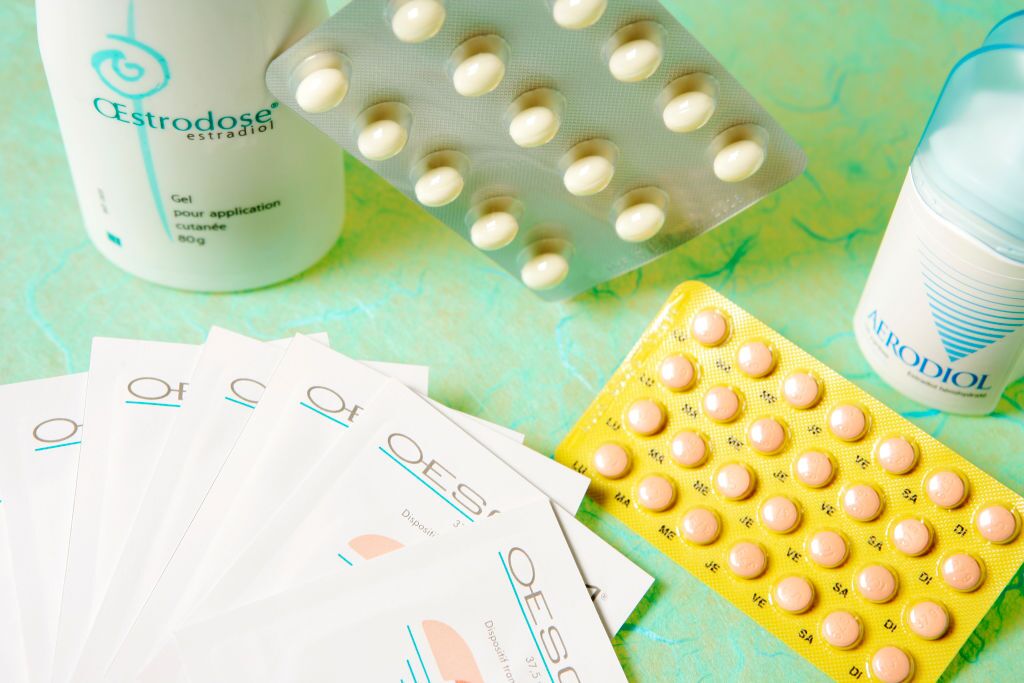
Examples of estradiol-based hormone therapies: Oesclim transdermal patch; Oestrodose dermal gel; Aerodiol nasal spray; Climaston tablets (estradiol and dydrogesterone); and progesterone capsules. (BSIP / UIG Via Getty Images)
This story was originally published by Katie Couric Media.
Inaccurate labeling and classification of menopausal hormone treatments have thwarted women’s access to quality healthcare for more than two decades. The good news? We are in the midst of major reform momentum. On Monday, Nov. 10, the U.S. Department of Health and Human Services announced that the Food and Drug Administration would eliminate the “boxed labeling” requirement for estrogen products. The “black box warning,” as it’s commonly called, is part of the fallout from a press conference that occurred more than 20 years ago, announcing the findings of the Women’s Health Initiative (WHI). It’s also been the subject of a half-century-long push and pull with the federal government.
The Backstory
When estrogen became all the rage in the 1970s, the FDA issued a mandate to provide stringent written warnings about its benefits and risks. At the time, this move was widely celebrated as a feminist win and a way to ensure women were armed with information to make decisions for themselves without medical gatekeeping. By the 1990s, the FDA initiated a more iterative labeling process for menopause treatments in order to keep up with new observational research. All that changed following the WHI’s misread of its data and the public frenzy it stoked. As a result, the FDA revisited the warning on estrogen, and in 2003 the agency upgraded the requirement to the most stringent “boxed labeling” format, which is applied to products that cause serious life-or-death reactions. In this case, the label warned of numerous conditions, including breast cancer, heart disease, blood clots and probable dementia. Apart from an increased risk in blood clots, none of the other warnings were statistically significant. (In medical research parlance, a non-statistically significant finding is not a finding.) Not surprisingly, the warning language and prominent placement caused undue alarm among patients and doctors alike. Usage plummeted—and a generation of women suffered.
Fast Forward to Today
With menopause advocacy gaining global attention, a modern campaign for better policies has emerged. Over the past year, the online citizen’s petition organized by Let’s Talk Menopause in support of removing the boxed warning from vaginal estrogen garnered more than 26,000 signatures. Nineteen states have introduced more than three dozen bills to improve menopause education and care. And the FDA, too, has now stepped up. In July, it convened a first-ever publicly broadcast roundtable discussion on menopausal hormone treatments; a dozen physicians presented, urging regulators to finally align policy with the data. The panel was followed by an open public comments and feedback period over several weeks. At Monday’s press conference and in a corresponding piece in JAMA, FDA commissioner Dr. Marty Makary shared that this process led the agency’s internal experts to determine that removing the boxed labeling requirement was the right thing to do. Make no mistake, this has been a longstanding demand—it’s neither new nor MAHA-driven. Doctors and scientists have made the case for its removal since the start to no avail, arguing the data from the WHI—the largest, most expensive, and only randomized placebo-controlled study of post-menopausal women—never supported putting it there in the first place.
.. . . .
For the here and now? Our advice is to trust the physicians, researchers and scientists who have advocated for this for decades regardless of who happened to be in charge of the FDA. This includes the Menopause Society and ACOG, among others. And importantly, don’t let polarization muddle the message. This is about policy, not politics. Our job is to continue to discuss hormone therapy the same way we would any medication. At long last, we are free to have this conversation without the unnecessary fear factor that the black box warning engendered.The FDA’s reversal of the labeling requirement is a major win for evidence-based medicine. Now it’s up to us to responsibly inform women of their choices.
https://msmagazine.com/2025/11/12/menopause-estrogen-fda-hormone-replacement-therapy-black-box-breast-cancer-women-health/
1 replies
 = new reply since forum marked as read
Highlight:
NoneDon't highlight anything
5 newestHighlight 5 most recent replies
= new reply since forum marked as read
Highlight:
NoneDon't highlight anything
5 newestHighlight 5 most recent replies
This FDA Decision Could Transform Menopause Care (Original Post)
niyad
Nov 15
OP
cachukis
(3,674 posts)1. On a roll today.