Environment & Energy
Related: About this forumRecovery of Lanthanide (Rare Earth) Elements and Fluorine From Molten Salt Electronic Slag.
The paper to which I'll briefly refer in this post is this one: Recovery of Rare Earth and Fluorine from Fluorinated Rare Earth Molten Salt Electrolytic Slag by Ball-Milling and Sodium Hydroxide Submolten Salt Processes Jie Tang, Jintang Liang, Liyuan Wang, Bing Yang, Guiping Huang, Jiachun Xiong, Qingqing Chang, Hailin Zhang, and Ping Li Industrial & Engineering Chemistry Research 2025 64 (17), 8665-8675.
Note: I prefer to use the formal term "lanthanides" for the common name, "rare earth elements" since the latter term, though popular, is somewhat misleading.
Recently I noted in this space concern about fluorine supplies: Fluorine Flows in China. A fluoride mineral, cryolite, which is now made synthetically for the manufacture of aluminum, is one of the first minerals (there will be more) to be considered extinct, having been depleted in mines in Greenland, an area under thread from the (I love this locution I learned and will steal it) apricot antichrist. Other fluorine minerals are being mined extensively. Interestingly, a lanthanide ore, bastnäsite, is a fluorine source as well as an ore for lanthanides.
Whatever.
Fluorine systems are used for the isolation of lanthanides from their ores.
A fun thing about this paper is that since the United States is in a free fall as it's being run by an idiot, the dollar is not the currency employed here with respect to cost. The currency is the Chinese Yuan, (RMB) which is equal to about 14 cents as of this writing.
From the introduction to the paper cited above:
The main challenge for the integrated recovery of FRMES is the phase dissociation of rare earth fluoride (REF3) in FRMES because it is difficult to dissolve in acidic and alkali environments under mild conditions. (5) The main methods currently used are acid roasting (sulfuric acid, (6) nitric acid, (7) etc.) and alkali roasting (sodium carbonate, (8) sodium hydroxide, (9) Borax, (10) etc.). Acid roasting mainly involves the reaction of REF3 to form soluble rare earth salts by adding concentrated acid and cosolvents at roasting temperatures above 360 °C. (11) However, the emission of volatile gases (HF, SOx, and NOx) and acidic wastewater caused potential environmental hazards. (12,13) Alkaline roasting adopts NaOH or Na2CO3 converting REF3 into rare earth oxides at high temperatures (greater than 700 °C) and then separating and recovering rare earths by water washing and hydrochloric acid dissolution. (1) Adding a CaO (14) or MgCl2 (15) fluoride fixer during the alkaline roasting process can cause the fluorine in REF3 to form acid-insoluble CaF2 or MgF2 for separation, respectively. Nevertheless, the incomplete conversion of REF3, high roasting temperature (greater than 700 °C), and low rare earth recovery still need to be improved. In addition, mechanochemical (5) and vacuum distillation treatment (16) processes are consistent with the concept of green environmental protection. There are problems such as small treatment capacity and high requirements for equipment, which make it difficult for them to realize large-scale applications in industry. Therefore, a simple, low-energy, and environmentally friendly technology for the recovery of rare earths from FRMES is required.
The sodium hydroxide submolten salt (SHSS) method demonstrates great advantages in the treatment of minerals and solid wastes, such as bauxite, (17) fergusonite, (18) zircon sand, (19) etc. Yang et al. (20) focused on the problem of high energy consumption in the recovery of rare earths from FRMES and utilized the SHSS method to treat FRMES at a NaOH concentration of 800 g/L and a reaction temperature of 200 °C, and the leaching efficiencies of rare earths and fluorine reached 99.05% and 98.23%, respectively. Yu et al. (21) adopted the SHSS method to recover rare earth elements in waste phosphors, and the leaching efficiencies of Ce and Eu reached 96.41% and 92.23%, respectively, under the conditions of a NaOH concentration of 700 g/L, reaction temperature of 200 °C, and reaction time of 4 h. Researchers utilizing SHSS to recover rare earth elements from rare earth solid waste required concentrated NaOH (greater than 700 g/L) to obtain a high leaching efficiency of rare earths. Liu et al. (5) utilized a mechanochemical method of adding NaOH (NaOH/smelting slag mass ratio 0.4/1) during ball milling to reduce the concentration of NaOH and achieved 96% leaching efficiency of REF3 from calcium-thermal reduction REF3 smelting slag (generated during the production of medium and heavy rare earth metals). These approaches achieved more than 95% leaching efficiency of rare earth from secondary rare earth resources by the SHSS method, while the high consumption of NaOH still required improvement. In addition, the reaction mechanism for extracting rare earths from FRMES by the SHSS method is unclear.
In this study, SHSS was utilized to dissociate REF3 in FRMES and recover rare earth elements and fluorine. The leaching process and kinetics of REF3 from ball-milled and unball-milled FRMES in the SHSS system were compared to elucidate the role of ball milling on the leaching mechanism of REF3. Subsequently, the leaching product, rare earth hydroxide, was purified and recovered by using HCl preferential dissolution and solvent extraction, with rare earth chloride as the final product. Finally, the process flow diagram was proposed, and the material balance and economic benefits were analyzed. This process has the advantages of low energy consumption, near-zero emission, and economy, which provides an approach for the comprehensive recycling of FRMES with the prospect of industrialized application...
The light lanthanides, up to gadolinium, are important in the manufacture of both electric motors and generators owing to the superior performance of neodymium iron boride magnets, which are generally stabilized with small amounts a heavy lanthanide, dysprosium. Things of which I do not necessarily approve, electric cars and wind turbines, involve the use of lanthanide based magnets.
A side note I can't avoid stating: I should, and will, note that used nuclear fuels are also lanthanide ores, as the light lanthanides are fission products, up to, and to a minor extent, including gadolinium. In fact, one form of nuclear reactor that is popular on the internet - although rare in actual practice - the thorium molten salt fluoride (FLIBE) reactor involves molten salt chemistry, although this is not what is being discussed in this paper. Of the specific elements found in used nuclear fuel, lanthanum, cerium, praseodymium, neodymium, and gadolinium will be exhibit low radioactivity after a short cooling period, the radioactive isotopes therein (for lanthanum and neodymium) being so long lived that they are found in the natural ores. Radioactive samarium and europium are suppressed by the fact that they are strong neutron absorbers and can, in fact, be used in control rods. Promethium does not occur on Earth, since all of its isotopes are radioactive and relatively short lived, but is found in used nuclear fuel, from which it can be isolated.
Anyway, the authors of this paper are working to minimize the environmental impact of lanthanide mining and isolation, while improving recovery efficiency.
The process involves, as demonstrated in the process flow diagram below, ball milling, heating in the presence of sodium hydroxide, washing with water to recover sodium fluoride, extracting the lanthanides in hydrochloric acid, and separation by traditional solvent extraction procedures.
Some figures from the text:
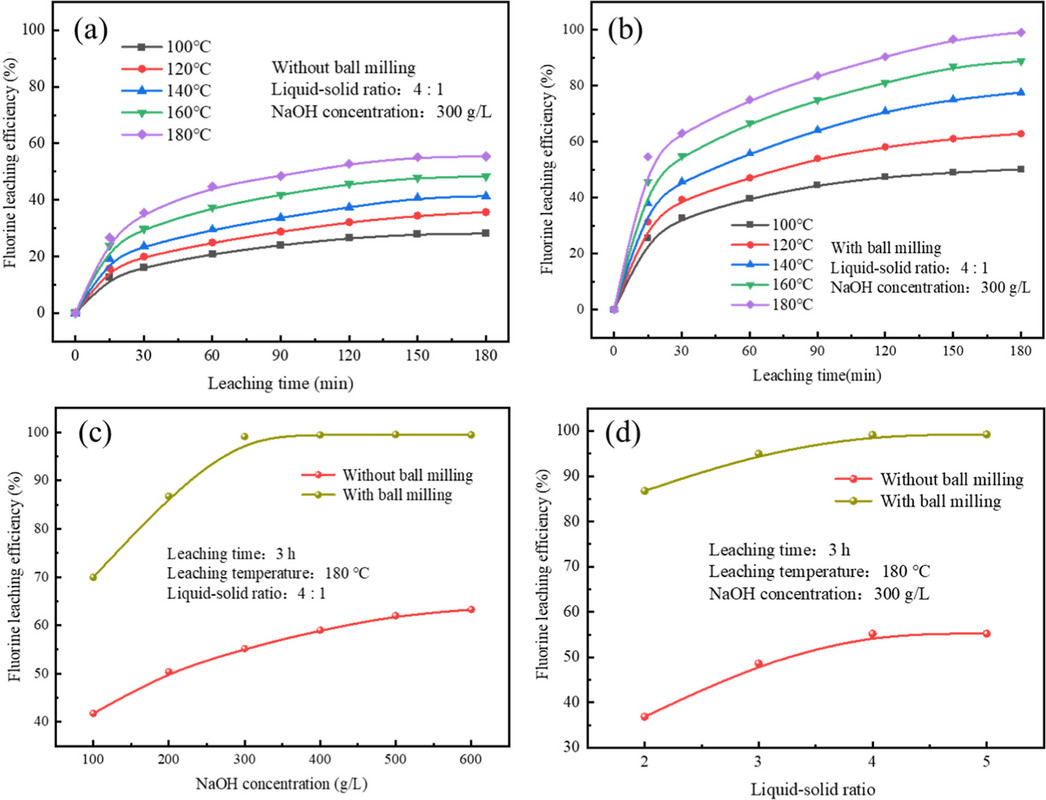
The caption:
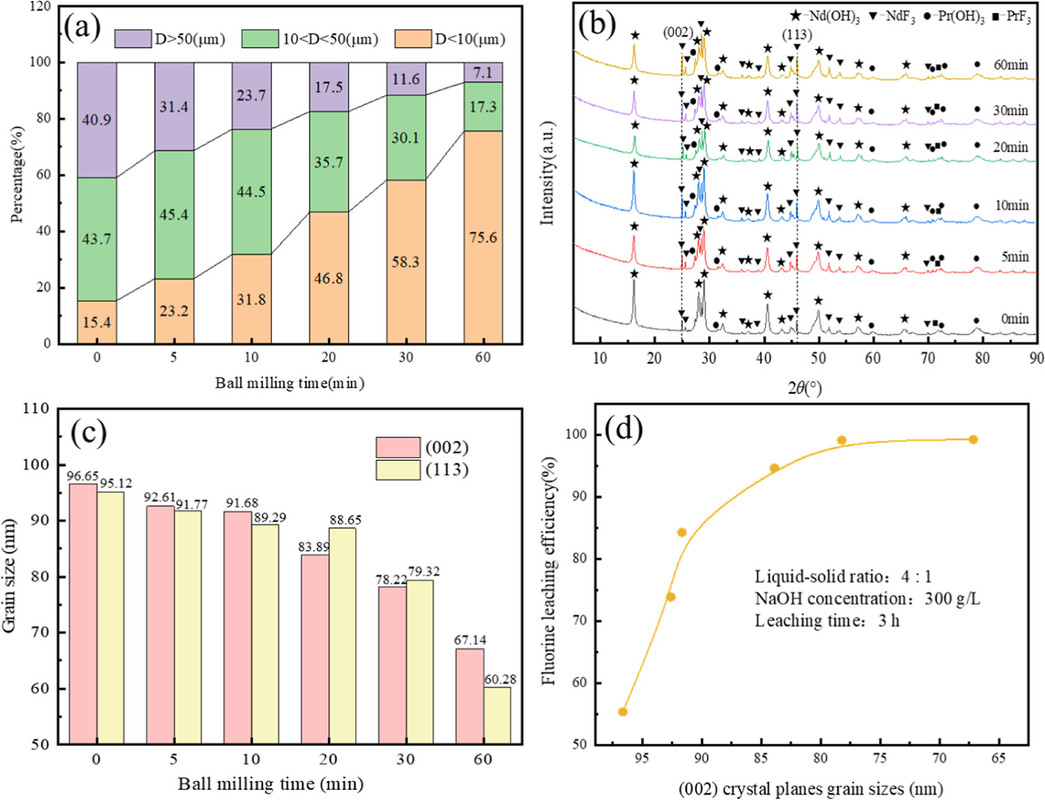
The caption:
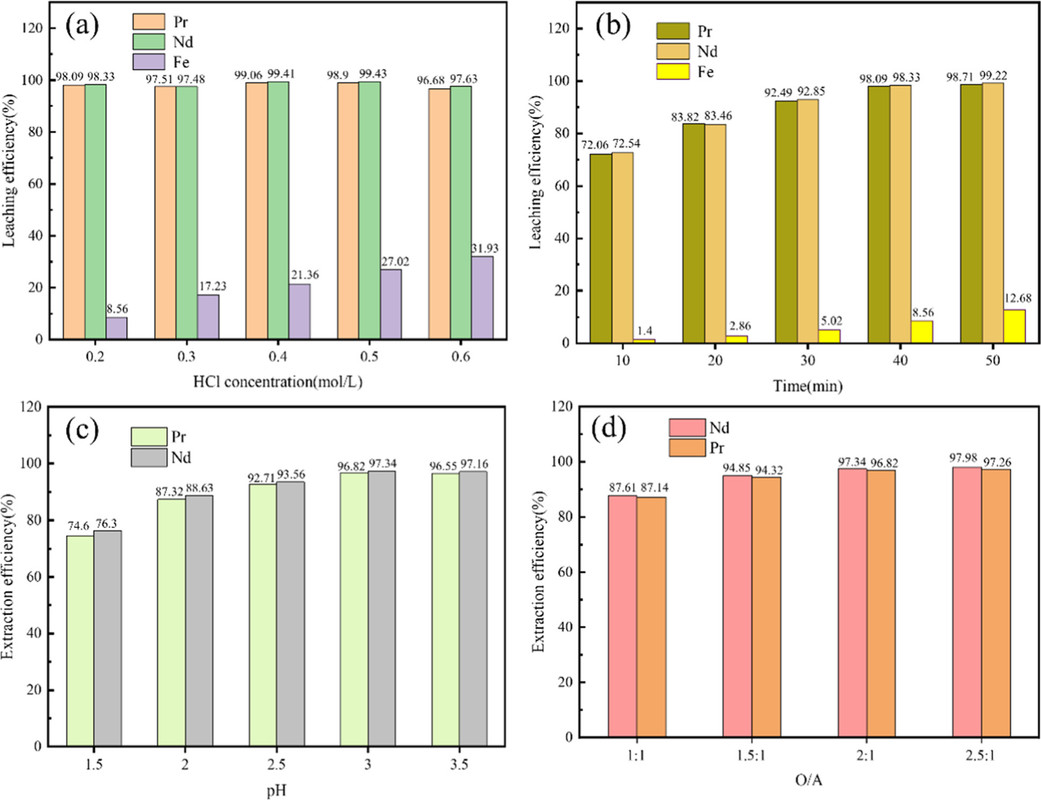
The caption:
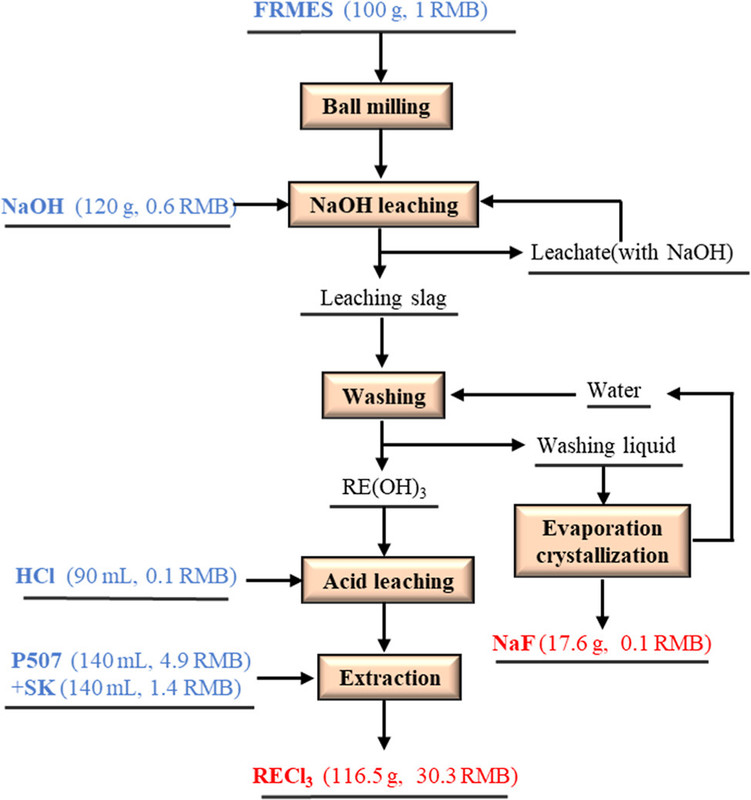
The caption:
From the paper's conclusion:
(1) REF3 can react spontaneously in the SHSS system. The dissociation efficiency of the REF3 phase in ball-milled FRMES was significantly improved, compared to that in unball-milled FRMES. The leaching efficiency of REF3 was 99.12% under the optimal leaching conditions of a NaOH concentration of 300 g/L, leaching temperature of 180 °C, leaching time of 3 h, and liquid–solid ratio of 4:1.
(2) The leaching process of REF3 in the SHSS system was mainly controlled by mixed chemical reaction and internal diffusion, and the apparent activation energies of REF3 were 35.57 and 41.31 kJ/mol, respectively, in ball-milled and unball-milled FRMES. Smaller grains size and more lattice defects in ball-milled FRMES than in unball-milled FRMES resulted in the exposure of more active sites and lower reaction activation energies and shorter reaction times.
(3) The recovery efficiencies of rare earths (Nd and Pr) exceeded 96% by HCl preferential dissolution and solvent extraction under the optimal conditions (HCl concentration of 0.2 mol/L, HCl leaching time of 40 min, initial aqueous pH of 3, and O/A of 2:1). 96.51% of fluorine was recovered. The gross profit per 100 g of FRMES processed was about 20.9 RMB, showing a good economic benefit.
I trust you'll have a pleasant weekend.